Novartis’ prostate cancer treatment has been approved by US Food and Drug Administration
25 March, 2022 | Pragati Singh
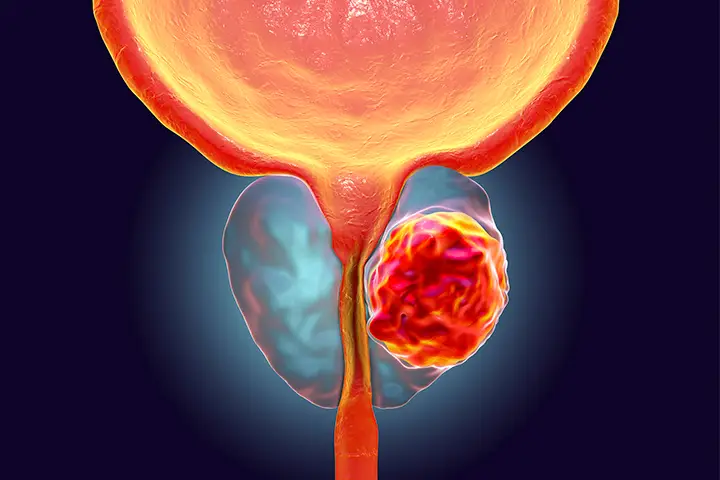
Novartis AG’s NOVN.S medication has been authorised by the US Food and Drug Administration for the treatment of patients with a kind of advanced prostate cancer that has spread to other regio...
Novartis AG’s NOVN.S medication has been authorised by the US Food and Drug Administration for the treatment of patients with a kind of advanced prostate cancer that has spread to other regions of the body, the pharmaceutical announced on Wednesday.
Novartis’ Pluvicto is a targeted radioligand therapy for adult patients who have already received anticancer treatment.
The treatment was purchased as part of the company’s $2.1 billion purchase of cancer pharmaceutical Endocyte in 2018.
According to Novartis, Pluvicto is a precision medicine that combines a targeted chemical, or ligand, with a cancer-killing radioactive particle.
Pluvicto has been filed for marketing permission to the European Medicines Agency and other health authorities, according to the business.
According to Novartis, two late-stage trials investigating Pluvicto in early lines of therapy for metastatic prostate cancer are now underway.










